Nitrosonium
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nitrilooxonium
| |||
| Systematic IUPAC name
Oxidonitrogen(1+)[1] | |||
| Other names
Nitrosonium
Iminooxidanium | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | NO(+) | ||
| ChEBI | |||
| ChemSpider | |||
| 456 | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
The nitrosonium ion is NO+, in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. It can be viewed as nitric oxide with one electron removed. This ion is usually obtained as the following salts: NOClO4, NOSO4H (nitrosylsulfuric acid, more descriptively written ONSO3OH) and NOBF4. The ClO−4 and BF−4 salts are slightly soluble in acetonitrile CH3CN. NOBF4 can be purified by sublimation at 200–250 °C and 0.01 mmHg (1.3 Pa).[2]
Synthesis and spectroscopy
NO+ is isoelectronic with CO, CN− and N2. It arises via protonation of nitrous acid:
- HONO + H+ ⇌ NO+ + H2O
In its infrared spectrum of its salts, νNO is a strong peak in the range 2150-2400 cm−1.[3]
Chemical properties
Hydrolysis
NO+ reacts readily with water to form nitrous acid:
- NO+ + H2O → HONO + H+
For this reason, nitrosonium compounds must be protected from water or even moist air. With base, the reaction generates nitrite:
- NO+ + 2 NaOH → NaNO2 + Na+ + H2O
As a diazotizing agent
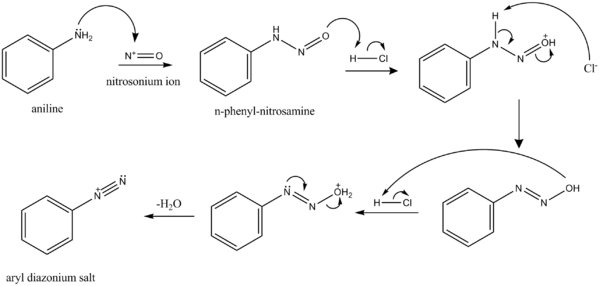
NO+ reacts with aryl amines, ArNH2, to give diazonium salts, ArN+2. The resulting diazonium group is easily displaced (unlike the amino group) by a variety of nucleophiles.
As an oxidizing agent
NO+, e.g. as NOBF4, is a strong oxidizing agent:[4]
- vs. ferrocene/ferrocenium, [NO]+ in CH2Cl2 solution has a redox potential of 1.00 V (or 1.46–1.48 V vs SCE),
- vs. ferrocene/ferrocenium, [NO]+ in CH3CN solution has a redox potential of 0.87 V vs. (or 1.27–1.25 V vs SCE).
In organic chemistry, it selectively cleaves ethers and oximes, and couples diarylamines.[5]
NOBF4 is a convenient oxidant because the byproduct NO is a gas, which can be swept from the reaction using a stream of N2. Upon contact with air, NO forms NO2, which can cause secondary reactions if it is not removed. NO2 is readily detectable by its characteristic orange color.
Nitrosylation of arenes
Electron-rich arenes are nitrosylated using NOBF4.[6] One example involves anisole:
- CH3OC6H5 + NOBF4 → CH3OC6H4NO + HBF4
Nitrosonium, NO+, is sometimes confused with nitronium, NO+
2, the active agent in nitrations. These species are quite different, however. Nitronium is a more potent electrophile than is nitrosonium, as anticipated by the fact that the former is derived from a strong acid (nitric acid) and the latter from a weak acid (nitrous acid).
As a source of nitrosyl complexes
NOBF4 reacts with some metal carbonyl complexes to yield related metal nitrosyl complexes.[7] In some cases, [NO]+ does not bind the metal nucleophile but acts as an oxidant.
- (C6Et6)Cr(CO)3 + NOBF4 → [(C6Et6)Cr(CO)2(NO)]BF4 + CO
See also
- Nitronium (NO2+)
- Nitric oxide (NO)
References
- ^ Nomenclature of Inorganic Chemistry : IUPAC Recommendations 2005 (Red Book). Cambridge: The Royal Society of Chemistry. 2005. p. 315. ISBN 978-0-85404-438-2.
- ^ Olah, George A.; Surya Prakash, G. K.; Wang, Qi; Li, Xing-ya; Surya Prakash, G. K.; Hu, Jinbo (15 October 2004). "Nitrosonium Tetrafluoroborate". Encyclopedia of Reagents for Organic Synthesis: rn058.pub2. doi:10.1002/047084289X.rn058.pub2. ISBN 0471936235.
- ^ Sharp, D. W. A.; Thorley, J. (1963). "670. The Infrared Spectrum of the Nitrosonium Ion". Journal of the Chemical Society (Resumed): 3557. doi:10.1039/JR9630003557.
- ^ N. G. Connelly, W. E. Geiger (1996). "Chemical Redox Agents for Organometallic Chemistry". Chem. Rev. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.
- ^ Williams, D. L. H. (1988). Nitrosation. Cambridge, UK: Cambridge University. pp. 21–22. ISBN 0-521-26796-X.
- ^ Bosch, E.; Kochi, J. K. (1994). "Direct Nitrosation of Aromatic Hydrocarbons and Ethers with the Electrophilic Nitrosonium Cation". Journal of Organic Chemistry. 59 (19): 5573–5586. doi:10.1021/jo00098a015.
- ^ T. W. Hayton, P. Legzdins, W. B. Sharp. "Coordination and Organometallic Chemistry of Metal-NO Complexes". Chemical Reviews 2002, volume 102, pp. 935–991.

